Abstract
Blinatumomab (BT), a CD19/CD3 bispecific T-cell engager (BiTE) antibody recently approved for treatment of relapsed/refractory acute lymphoblastic leukemia (r/r ALL), has resulted in a 40-50% complete response (CR)/CR with incomplete count recovery (CRi) rate and frequent cytokine release syndrome (CRS) events. While extent of disease burden has been identified as a key predictor of disease response, there have been no studies to date evaluating the impact of single nucleotide polymorphisms (SNPs) in cytokine genes on disease response or CRS after treatment with BT. Here, we evaluated the possible association between cytokine SNPs, disease response, and/or CRS in r/r ALL patients with receiving BT.
A total of 82 patients (consecutive case-series) received BT between 2012 and 2017 at City of Hope. Of these, 68 patients had archived DNA samples available for study and analysis. Genotyping was done using Illumina's Omni 5-4 bead array, which interrogates more than 4.2 million SNPs. Array processing was done following manufacturer's recommendations and arrays were scanned by Illumina's iScan instrument. Beeline software and Genome Studio (Illumina) were used for analysis of scanned images and to generate the genotyping calls and quality control reports, respectively. Our analysis focused on the following cytokine genes of interest (known to be associated with autoimmune diseases/graft-versus-host disease): IL-1B, IL-2, IL-6, IL-7R, IL-10, IL-11, IL-12, IL-17A, IL-23R, TNF-α, TGF-β, and IFN-γ. Two modes of inheritance (dominant and recessive) were considered whenever appropriate. Univariate analyses of SNPs, response and CRS were performed using Fisher's exact test. P values were not adjusted for multiple hypothesis testing due to the exploratory nature of the analyses.
In our cohort, median age of patients at BT treatment was 34 years old (range: 7-85), with 32 females (39%) and 50 males (61%). Patients' race/ethnicity included 49 Hispanic (60%), 20 non-Hispanic Caucasians (24%), 9 Asians (11%), and 3 African Americans (3%). All patients had primary refractory (n=16) or relapsed disease status (1st relapse: n=38, 2nd or subsequent relapse: n=27) with median lines of prior therapy of 2 (range: 0-5). Cytogenetic abnormalities included Ph+ALL in 11 patients, while 50 patients had other cytogenetic abnormalities and 15 had normal cytogenetics. Bone marrow blasts of >50% at BT treatment was detected in 45 patients, and 22 patients had a history of or active extra-medullary involvement. After BT treatment, 37 patients (45%) achieved CR/CRi with a median duration of 9.5 months (range: 1-37), 54 patients (66%) experienced CRS (grade 1: n=35, grade 2: n=14, and grade 3: n=5), and 9 patients developed neurotoxicity. Lastly, the peak C-reactive protein level (median/range) was 17 mg/L (range: 1-392).
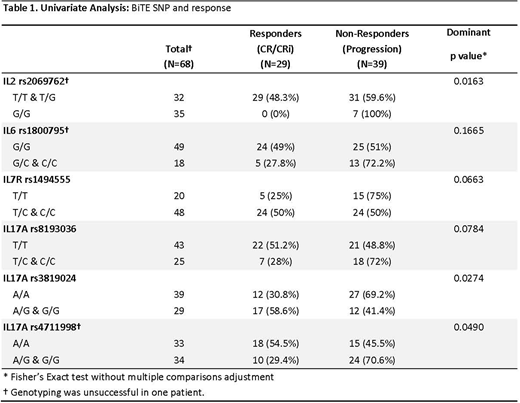
By univariate analysis, disease response (CR/CRi) to BT was significantly associated with the following cytokine SNPs: IL2 rs2069762 [48.3% (T/T-G/T: n=60) Vs. 0% (G/G; n=7), p=0.016], IL17A rs3819024 [30.8% (A/A; n=39) Vs. 58.6% (A/G-G/G; n=29), p=0.027], and IL17A rs4711998 [54.5% (A/A: n=33) Vs. 29.4% (A/G-G/G: n=34), p=0.049]. (Table 1) These three cytokine SNPs and two additional SNPs with p-values between 0.05 and 0.1 (IL7R rs1494555, IL17A rs8193036) were evaluated by multivariable analysis, adjusted for >50% bone marrow blasts, which was associated with lower rate of CR/CRi (33% vs. 63% with <50%, p=0.02). By multivariable analysis, IL17A rs3819024 remained significant while IL17A rs4711998 was at borderline significance (p=0.06). (IL2 rs2069762 could not be tested due to no CR/CRi occurrence). None of the cytokine SNPs analyzed were associated with CRS (0 vs. any grade, or grades 0-1 vs. 2-3).
In conclusion, to our knowledge this is the first study to demonstrate a possible association between treatment response to BT and cytokine genetic polymorphisms. Our hypothesis-generating data suggest a potential role of IL17 and IL2 in BT response and justify a larger confirmatory study, which may lead to personalized BT immunotherapy for B-ALL.
Stein:Amgen Inc.: Speakers Bureau; Celgene: Speakers Bureau. Forman:Mustang Therapeutics: Other: Licensing Agreement, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal